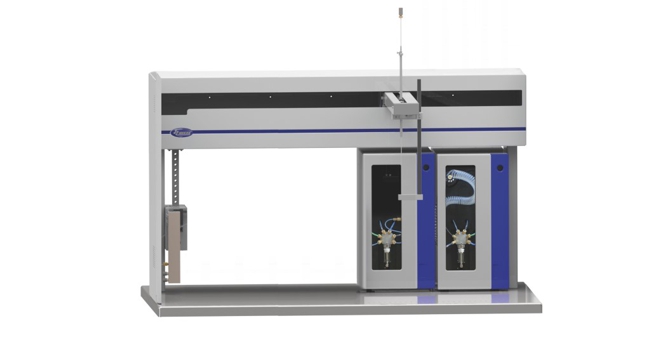
The GPC Cleanup Module performs Automated Gel Permeation Chromatography (GPC) for the cleanup of a wide range of sample matrices including foods, tissues, grains, plants and environmental samples such as soil, sludge, and hazardous waste for EPA (SW-846 Method 3640A), CLP, FDA, USDA, USDI and DFG S 19 (L00.00-34, DIN EN 12393) analysis.
Gel Permeation Chromatography
Gel Permeation Chromatography refers to displacement of a solution containing analytes of interest, using a mobile (solvent) phase, through a bed of porous particles. In most applications for GPC cleanup of organic extracts, the porous column bed, which is the stationary phase, if composed of cross‐linked polymeric material. A classic example is polystyrene that has been polymerized with a small amount of divinylbenzene. The resulting beads are swollen with solvent to form a “gel” that can be packed into a column.
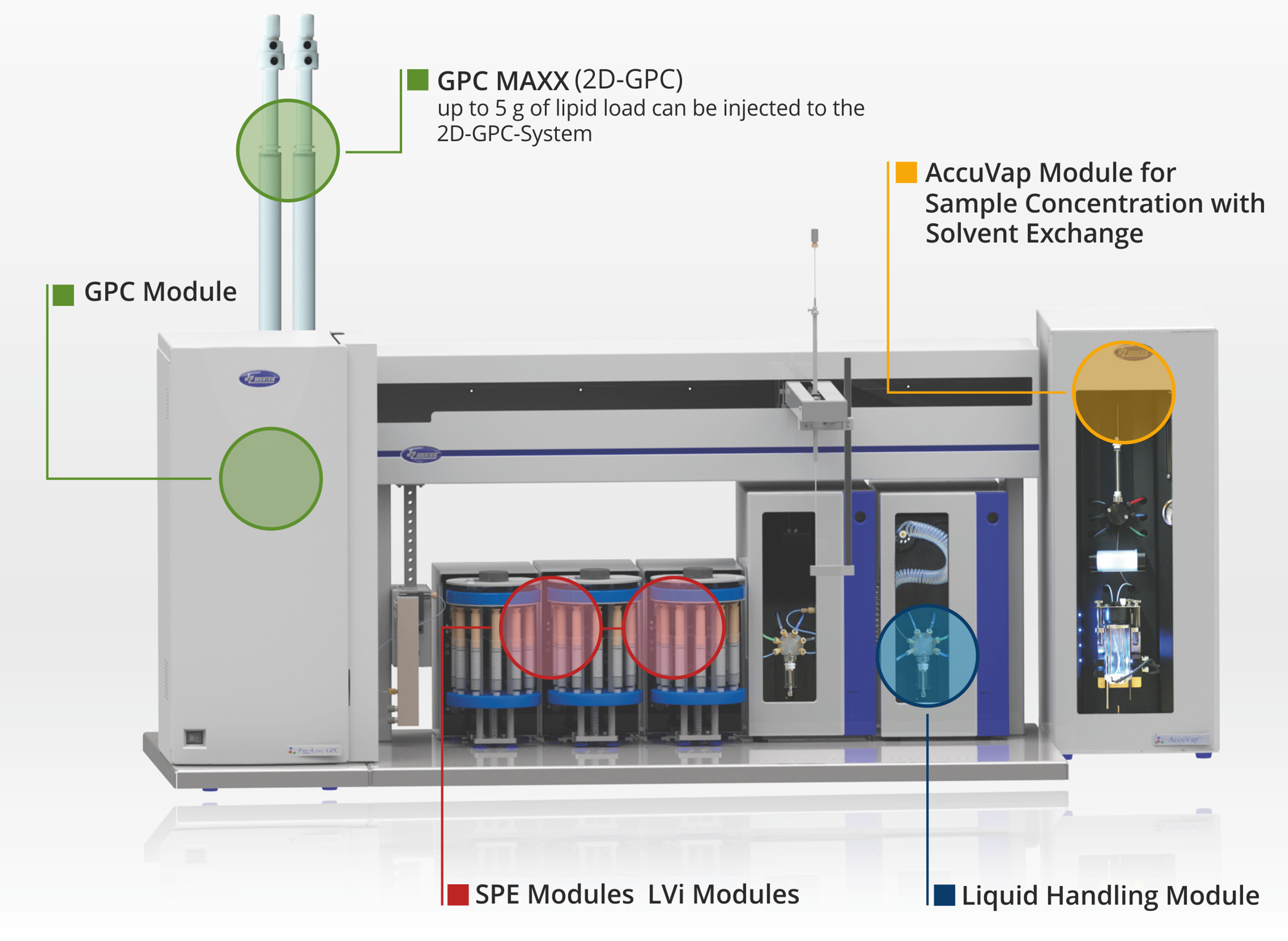
PrepLinc - the modular system for automated sample cleanup
Using our experience with GPC Cleanup, J2 Scientific has perfected it on this new platform. All the unique features of previous models are combined with high-powered software and the ability for further automation by combining GPC with other prep processes on the PrepLinc™.
The PrepLinc™ GPC Cleanup Module provides another level of functionality to the PrepLinc™ system. The ability to combine GPC Cleanup with Automated Concentration and/or SPE on the PrepLinc™ system, completes the sample prep package.
PrepLinc GPC Cleanup Module
Standout Features:
- Direct Inject - inject the entire sample onto the column
- Septum piercing standard for sample & collect vials
- Probe tracking & user-defined rinses
- 2- or 5-column selector valve
- UPS Power Watchdog
- Sample Dilution
- Multiple Injections
- Specialized calibration & reporting software features for GPC
- Create methods directly from the column calibration file
- Use with low-pressure glass or high pressure columns
- Integrate with PrepLinc SPE and AccuVap modules

GPC Method Editor
- Programmable dilution prior to injection
- Different flow rate during equilibration
- User defined rinses
- Collect fraction flow directed to SPE column
- Collect fration flow directed to AccuVap
- Program multiple collect
- fractions; direct each to vial, SPE or AccuVap individually
GPC Cleanup Reports
The PrepLinc™ software makes keeping track of your column calibration and sample data easy. Create Method and Configuration reports to report sytsem setup. Create Sequence reports with time/date stamps. Create Calibration and Comparison reports in compliance with Method 3640a and CLP guidlines. Quickly determine if a column calibration has met pass/fail requirements and print a report to prove it!
Autosampler Options
Choose the autosampler size based on desired batch capacity and level of integration with other PrepLinc modules. Choose Model AS2 autosampler for capacities up to 72 samples per batch and integration with up to two SPEi column modules. Choose Model AS4 autosampler for capacities up to 200 and full SPEi integration.
PrepLinc Module Options
Downloads
Application Notes
Application Note 105
Gel Permeation Chromatography is a size-exclusion liquid chromatography method used to remove lipids, sulfur and other co-extractives from environmental and food matrices prior to analytical analysis. It is a desirable technique because it is non-destructive and separates based on molecular size.
GPC Cleanup, while a beneficial cleanup technique, has been criticized for its solvent and time investments. GPC Cleanup using the traditional glass column requires one hour per sample and about 300mL of mobile phase solvent. Additionally, the traditional mobile phase is methylene chloride (DCM), a chlorinated solvent that requires expensive disposal.
To significantly decrease the cost of GPC Cleanup, the run time must be shortened. Simply increasing the mobile phase flow rate may speed the processing time, but will not decrease the amount of solvent used. It will also create pressure problems with the column. Another way to increase sample throughput while decreasing solvent consumption is to decrease the bed volume of the column. The lower bed volume will decrease the run time, thus decreasing the amount of solvent used to process each sample. There are, however, drawbacks to decreasing column bed length in some situations.
Please request your copy at J2 Scientific GmbH.
Application Note 110
The USDA FSIS National Residue Program mandates the testing of domestic meat to prevent violative levels of persistent pollutants like chlorinated pesticides from entering the food supply. Recent findings have prompted an interest in flame retardant levels in meat. Flame retardant compounds, like hexabromobiphenyl, are commonly found in flame retardants and enter the animal by ingestion of retardant-treated items. Little is known of the toxicity of fire retardant compounds in humans, but research in rodents suggests they are associated with cancer, endocrine disruption and brain impairment. Like chlorinated pesticides, fire retardant compounds are highly lipophilic and tend to accumulate in fatty tissue of animals in the food chain.
The standard method for determining chlorinated pesticide residue levels in meat employs GPC Cleanup with GC/ECD detection. In this study the flame retardant compound hexabromobiphenyl was simultaneously determined with a standard list of 20 chlorinated pesticides. Advances in GPC Cleanup column technology allows for a decrease in run time, keeping the entire procedure, extraction through analysis, close to 1 hour per sample.
Please request your copy at J2 Scientific GmbH.
Application Note 132
Currently, 12 substances are regulated by the Stockholm Convention on Persistent Organic Pollutants (POPs) signed in May 2001 by 127 countries, and the work on finding new candidate chemicals to the convention has started. One group of substances in focus is polycyclic aromatic hydrocarbons (PAHs). They are formed during all types of incomplete combustion of organic matter, and they exhibit the characteristic POPs properties: persistence, bio-accumulation, adverse effects and potential for long-range environmental transportation to a certain extent. Many of the PAHs are carcinogenic, they are also believed to exhibit reproductive effects, as well as immune system inhibiting properties, genotoxicity and mutagenicity.
The development of innovative analytical methods for determination of PAHs has been and is of fundamental importance, due to the high carcinogenicity of these compounds. The quali-quantitative analysis of PAHs is an important challenge due to the low concentration at which these hydrocarbons may be present.
Please request your copy at J2 Scientific GmbH.
Application Note 131
The use of Dietary Supplements by consumers has grown from <10% of the population to ~50% of the population over 10 years (US). Ginseng, one of the most popular botanical supplements, is a root crop requiring 4-7 years to mature. The long growing period increase the risk of fungal and insect attack. Numerous chlorinated pesticides, namely PCNB (Quintozene) and Tricyclazole are frequently found in ginseng samples. FDA & private laboratory testing revealed major contamination problems in the 1990’s which still persist today making residue monitoring a high priority. While many supplements can be routinely tested using modern techniques (QuEChERS), many chlorinated residues and high-lipid/saponin matrices can create challenges for this modern technique. Florisil column clean up (SPE) and Gel Permeation Chromatography (GPC) are utilized for such matrices. Historical GPC involved significant time and resources from the analyst to collect fractions, evaporate them and perform various solvent exchanges & SPE. Using the new-generation PrepLinc™ system, these functions are fully automated creating a ready-to-inject sample in an autosampler vial making GPC viable again for production laboratories. This poster (the first in a series) will introduce the system and briefly outline the methods utilized for sample preparation & provide matrix examples. Future posters/papers will provide further detail on the project.
Please request your copy at J2 Scientific GmbH.
Application Note 120
Extract purification for pico- or nanogram scale GC/MS is time consuming, laborious and costly, and may suffer from performance variations in manual cleanup chromatography. Size exclusion (GPC) followed by adsorptive chromatography is useful for cleanup of biota, soil and sediment extracts for high resolution PCDD/PCDF analyses. GPC cleanup of extracts is allowed or encouraged in several EPA methods and is of great value to laboratories practicing such analyses. Sample extract cleanup for PCDD./PCDF always involve a carbon column stage. Sample concentrate, typically after other cleanups, is passed onto carbon and forward elution drives out various interferences. Reverse elution utilizes solvent containing a component (e.g. toluene) having great affinity for carbon. This disgorges PCDD/PCDF congeners into an eluate ready for evaporation and analysis.
In this study GPC eluate was forward eluted through a carbon cartridge (bed of powder mixed with granular substrate, packed between two frits) placed in line after the GPC column. During forward elution, target compounds collect at or near the column head while interferences are flushed forward. Valve switching enables reverse elution with toluene for collection of targets. Thus PCDD/PCDF sample cleanup can be conducted in highly automated fashion with minimal operator contact.
Please request your copy at J2 Scientific GmbH.
Application Note 30
Chlorination of drinking water has been a commonly practiced method of disinfection for over a century. However, the disinfection by-products arising from the reaction of organic material in the water and chlorine may give rise to certain aberrant carcinogenic effects. EPA Method 552.1 and 552.3 prescribe procedures for testing of haloacid constituents in drinking water by ion-exchange or micro extraction, followed by esterification and quantitation by GC-ECD.
In this study, the 552.1 ion-exchange method is automated for different water samples to afford increased reproducibility, unattended operation, and consistency of sample loading and elution.
Please request your copy at J2 Scientific GmbH.
- Maximizing Lipid Load With 2-Dimensional GPC Cleanup
- QuEChERS, SPE and GPC: A Comparison of Sample Preparation Techniques for Analysis of Pesticides in Problematic Matrices
- Automated GPC with Inline SPE to Improve Sample Cleanup Without Adding Time or Solvent
- Additional cleanup for DIN EN 12393 minimising matrix effects and improving result quality in GC-MS
- A Combined SPE Method for Analysis of Chloroacetic Acids in Drinking Water
- Bestimmung von leichten und schweren polycyclischen aromatischen Kohlenwasserstoffen (PAKs) in Fetten und Ölen pflanzlicher und tierischer Herkunft mittels automatisierter 2D-GPC und anschließender GC-MS-Detektion
- Automatisierte Probenvorbereitung für die Bestimmung von Pestizidrückständen in hocheffizienten Laboratorien mit GPC-GC-MS/MS und -LC-MS/MS
- Maximierte Probenaufgabe mit zweidimensionaler Gelpermeationschromatografie (2D-GPC)
- Automatisierung der Bausteine GPC, C1 und C2 der Methode L 00.00-34
- Cleanup-Methode für Nahrungsergänzungsmittel wie z.B. Ginseng
- Modifizierte Cleanup-Methode für Dioxine und persistente organische Schadstoffe (POPs)
- Automatisierung der Wasserextraktion mit dem SPE-Wasserextraktionssystem LVi
- Traditionelles Dioxin-Cleanup mit dem PrepLinc System
- Wiederauffindungsraten unterschiedlicher Pflanzenschutzmittel mit GPC und AccuVap
- Erfahrungen zur Automatisierung des GPC-Reinigungsver fahrens bei der Untersuchung von tierischen Lebensmitteln auf Chlorkohlenwasserstoffe
- Der Einsatz der automatischen Gelchromatographie zur Reinigung von Pesticidextrakten Organochlor
- Pflanzenbehandlungsmittel in Tabak und Tabakerzeugnissen
- Bestimmung der Fungizide Bitertanol, Fuberidazol, Imazalil, Rabenzazole, Triadimefon und Triadimenol in Pflanzen und Boden
- Methode zur Aufarbeitung von Lebensmitteln und Futtermitteln pflanzlicher und tierischer Herkunft für die Multirückstandsbestimmung lipoid- und wasserlöslicher Pflanzenbehandlungsmittel
- Zur Analytik von Chlorkohlenwasserstoffen in Zwiebeln nach Reinigung mit der Gelpermeationschromatographie
- Schnelle Untersuchung von Milch auf chlorierte Kohlenwasserstoffe mittels automatischer Gelchromatographie
- Automatisierte Gelchromatographie als Reinigungsverfahren zum Nachweis von ECD-erfaßbaren Wirkstoffen, chlorierten Kohlenwasserstoffen, Pentachlorphenol sowie von Diphenyl und o-Phenylphenol in pflanzlichen Materialien
- Untersuchungen zum Einsatz der Gelpermeationschromatograpie in der Rückstandsanalytik
- Nachweis von Aflatoxin B1 in Futtermitteln für Milchtiere
- Bestimmung der Rückstände von aromatischen Dinitroverbindungen mittels gelchromatischer Reinigung
- Die Gelpermeationschromatographie, eine universelle Reinigungsmethode in der Analytik von Pflanzenschutzmitteln
- Untersuchungen zur Messung und Bewertung von Rückständen des Ektoparasitenbekämpfungsmittels Phoxim in Milch
- Methode zur Aufarbeitung von Lebensmitteln und Futtermitteln pflanzlicher und tierischer Herkunft für die
- Multirückstandsbestimmung lipoid- und wasserlöslicher Pflanzenbehandlungsmittel
- Untersuchungen zur Gelchromatograpie (GPC) als Reinigungsverfahren in der Rückstandsanalytik von Tierarzneimitteln
- Eine schnelle Methode zur Bestimmung des Ebergeruchsteroids Androstenon
- Analysenverfahren zur Bestimmung von polychlorierten Dibenzodioxinen und Dibenzofuranen in Frauenmilch
- Untersuchungen zum Vorkommen von Moschus-Xylol in Fischen
- GPC-clean up von fetthaltigen Matrizes in der Rückstandsanalytik unter Verwendung von OPTIMA-Säulen
- Entwicklung einer Methode zur Bestimmung von Nitromoschusverbindungen im Hausstaub
Contact
J2 Scientific GmbH
Reifenstuelstraße 3
80469 Munich / Germany
Tel.: +49 (0) 89 72069587
E-Mail: info@j2scientific.eu
Distributor
ANTEC GmbH
Analysen- und Prozesstechnik
Hauptstraße 4
82404 Sindelsdorf
Tel.: +49 (0) 8856 9910